
Pipeline
Relapsed/Refractory: Combination with Standard of Care
Watch & Wait setting: Monotherapy
OncoMimics™
A potential major breakthrough in cancer immunotherapy produced using our Mimicry.
OncoMimics™ are a specific combination of bacterial peptides, derived from bacteria present in the gut microbiome, that closely mimic either overexpressed tumor-associated antigens (TAAs) or lineage-specific markers in solid and liquid tumors, respectively.
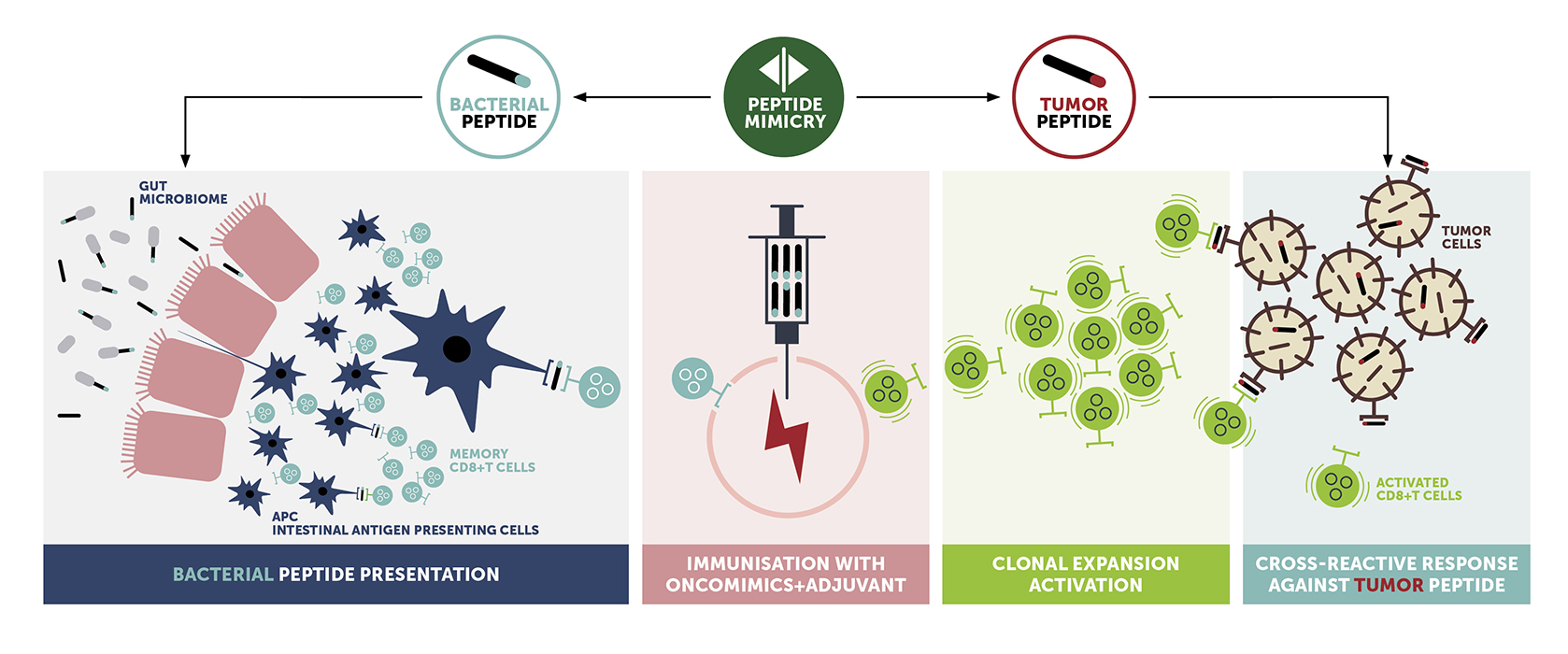
Key differentiating factors of OncoMimics™ immunotherapies include:
- Expected faster, stronger and durable immune responses
- Enterome uses non-self bacterial peptides to target tumors which allow us to tap into a pool of memory T cells with pre-existing immune response
- Excellent immunogenicity
- Enterome uses the highest possible affinity binding peptides (<10nM) derived from our extensive bacterial protein database
- Ability to counteract tumor heterogenicity
- Enterome uses multiple high-affinity peptides in each product targeting multiple key TAAs
- Expected good safety profile
- Tumor expression drives OncoMimics™ peptides selection
- Potential to access broad patient populations (off-the-shelf)
- De-risked Chemistry, Manufacturing and Control (CMC) and regulatory paths
EndoMimics™
New generation of oral bioactives to treat inflammatory and autoimmune diseases generated from our Mimicry drug discovery platform.
EndoMimics™ are first-in-class orally available bioactives drugs based on proteins secreted by the gut bacteria that act like human hormones or cytokines.
EndoMimics™ drug candidates generated are expected to have the following advantages:
- Novel mode of action based on mimicking the effects or actions of specific hormones or cytokines
- Potential to treat various immune diseases by accessing a wide range of cellular targets
- Ability to be fast tracked from screening to preclinical candidate given there is no need for optimization from hit to clinical candidate
- Excellent safety profile given that microbiome-derived molecules are naturally present in humans leading to the development of low immunogenicity and good tolerance
- Local delivery and local efficacy as they will be administered in a tailored formulation designed to target release in specific GI region
Enterome and Nestlé Health Science will co-develop Enterome’s lead EndoMimics™ compound, EB1010, targeting food allergies and inflammatory bowel disease (IBD).
AllerMimics™
New generation of therapeutics to address Food Allergies
AllerMimics™ are a new generation of therapeutics that leverage Enterome’s unique Mimicry scientific concept. AllerMimics™ have an immunomodulator mechanism of action that induce tolerogenic allergen-specific responses via food epitope mimicry.
Enterome aims to adapt its existing pipelines of microbiome-derived MHC-II restricted allergen mimics and in particular target
- AllerMimics™- that induce specific Tregs
- Allergen-specific deactivation of Th2 cell function (T cell anergy)
AllerMimics™ are designed to switch off allergic reactions to allergens by targeting the underlying cause of disease. The AllerMimics™ approach has the potential to expand to the treatment of any allergy based on a specific allergy-related allergen peptide and to multi-food allergies based on addressing a range of specific food peptides.
Partnered Products
Nestlé Health Science Partnering Programs
The aims for the collaboration are to:
- Successfully develop and commercialize Enterome’s lead EndoMimics™ pipeline candidate EB1010. EB1010 is a potent local inducer of IL-10 designed to provide improved therapeutic outcomes for patients with food allergies and IBD. EB1010, which is due to enter clinical trials in 2023, was discovered using Enterome’s novel bacterial Mimicry drug discovery platform. The same platform will also be used to identify and develop new EndoMimics™ as potential novel therapies for inflammation associated with food allergies.
- Identify and create a pipeline of novel AllerMimics™ (allergen immunotherapies for food allergies) using Enterome’s highly productive Mimicry platform with an initial focus on peanut allergens as the basis for a novel class of immunotherapies that aims to suppress allergic reactions.